Acid Dissociation Constant on:
[Wikipedia]
[Google]
[Amazon]
 After rearranging the expression defining ''K''a, and putting , one obtains
:
This is the
After rearranging the expression defining ''K''a, and putting , one obtains
:
This is the
 A polyprotic acid is a compound which may lose more than 1 proton. Stepwise dissociation constants are each defined for the loss of a single proton. The constant for dissociation of the first proton may be denoted as ''K''a1 and the constants for dissociation of successive protons as ''K''a2, etc.
A polyprotic acid is a compound which may lose more than 1 proton. Stepwise dissociation constants are each defined for the loss of a single proton. The constant for dissociation of the first proton may be denoted as ''K''a1 and the constants for dissociation of successive protons as ''K''a2, etc. 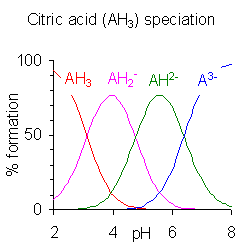 When the difference between successive p''K'' values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
According to Pauling's first rule, successive p''K'' values of a given acid increase . For oxyacids with more than one ionizable hydrogen on the same atom, the p''K''a values often increase by about 5 units for each proton removed, as in the example of phosphoric acid above.
It can be seen in the table above that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it. That why p''K''a2 is greater than p''K''a1. p''K''a3 is greater than p''K''a2 because there is further charge separation. When an exception to Pauling's rule is found, it indicates that a major change in structure is also occurring. In the case of (aq), the vanadium is
When the difference between successive p''K'' values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
According to Pauling's first rule, successive p''K'' values of a given acid increase . For oxyacids with more than one ionizable hydrogen on the same atom, the p''K''a values often increase by about 5 units for each proton removed, as in the example of phosphoric acid above.
It can be seen in the table above that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it. That why p''K''a2 is greater than p''K''a1. p''K''a3 is greater than p''K''a2 because there is further charge separation. When an exception to Pauling's rule is found, it indicates that a major change in structure is also occurring. In the case of (aq), the vanadium is
AH2+ <=> AH + H+ \qquad HH+] = \mathit_1 H2+/chem>
: AH <=> A- + H+ \qquad - H+] = \mathit_2 H/chem>
Substitute the expression for Hfrom the second equation into the first equation
: - H+]^2 = \mathit_1 \mathit_2 H2+/chem>
At the isoelectric point the concentration of the positively charged species, , is equal to the concentration of the negatively charged species, , so
:
Therefore, taking
B + H2O <=> HB+ + OH-
Using similar reasoning to that used before
:
''K''b is related to ''K''a for the conjugate acid. In water, the concentration of the
H2CO3 + H2O <=> HCO3- + H3O+
but also the conjugate acid of the HCO3- + OH- <=> CO3^2- + H2O
Carbonic acid equilibria are important for NH2CHRCO2H <=> NH3+CHRCO2-
At pH less than about 5 both the carboxylate group and the amino group are protonated. As pH increases the acid dissociates according to
: NH3+CHRCO2H <=> NH3+CHRCO2- + H+
At high pH a second dissociation may take place.
: NH3+CHRCO2- <=> NH2CHRCO2- + H+
Thus the amino acid molecule is amphoteric because it may either be protonated or deprotonated.
H2O <=> OH- + H+
where in aqueous solution denotes a solvated proton. Often this is written as the
HCl + CH3CO2H <=> Cl- + CH3C(OH)2+
:
Compare this reaction with what happens when acetic acid is dissolved in the more acidic solvent pure sulfuric acid:
:H2SO4 + CH3CO2H <=> HSO4- + CH3C(OH)2+
 The unlikely
The unlikely
 When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine p''K''a values in a solvent mixture such as water/
When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine p''K''a values in a solvent mixture such as water/

 With organic acids inductive effects and
With organic acids inductive effects and
 The experimental determination of p''K''a values is commonly performed by means of
The experimental determination of p''K''a values is commonly performed by means of
 For some molecules, dissociation (or association) can occur at more than one nonequivalent site, and the observed macroscopic equilibrium constant or macroconstant is a combination of microconstants involving distinct species. When one reactant forms two products in parallel, the macroconstant is a sum of two microconstants, This is true for example for the deprotonation of the
For some molecules, dissociation (or association) can occur at more than one nonequivalent site, and the observed macroscopic equilibrium constant or macroconstant is a combination of microconstants involving distinct species. When one reactant forms two products in parallel, the macroconstant is a sum of two microconstants, This is true for example for the deprotonation of the  Similarly, a base such as
Similarly, a base such as
Acidity–Basicity Data in Nonaqueous Solvents
Extensive bibliography of p''K''a values in dimethyl sulfoxide, DMSO, acetonitrile, THF, heptane, 1,2-dichloroethane, and in the gas phase
Curtipot
All-in-one freeware for pH and acid–base equilibrium calculations and for simulation and analysis of potentiometric titration curves with spreadsheets
SPARC Physical/Chemical property calculator
Includes a database with aqueous, non-aqueous, and gaseous phase p''K''a values than can be searched using Simplified molecular input line entry specification, SMILES or CAS registry numbers
Aqueous-Equilibrium Constants
p''K''a values for various acid and bases. Includes a table of some solubility products
Explanations of the relevance of these properties to pharmacology
Free online prediction tool (Marvin)
p''K''a, log ''p'', log ''d'' etc. From Chemaxon, ChemAxon * Chemicalize.org:Chemicalize.org#List of the predicted structure based properties, List of predicted structure based properties * p''K''a Char
by David A. Evans {{Chemical solutions Equilibrium chemistry Acids Bases (chemistry) Analytical chemistry Physical chemistry
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a HA <=> A^- + H^+
known as
HA <=> A- + H+
The equilibrium constant for this dissociation reaction is known as a dissociation constant. The liberated proton combines with a water molecule to give a hydronium (or oxonium) ion (naked protons do not exist in solution), and so Arrhenius later proposed that the dissociation should be written as an HA + H2O <=> A- + H3O+
 Brønsted and Lowry generalised this further to a proton exchange reaction: Includes discussion of many organic Brønsted acids.
Chapter 5: Acids and Bases
:
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide: the solvent S acts as a base, accepting a proton and forming the conjugate acid .
:
Brønsted and Lowry generalised this further to a proton exchange reaction: Includes discussion of many organic Brønsted acids.
Chapter 5: Acids and Bases
:
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide: the solvent S acts as a base, accepting a proton and forming the conjugate acid .
:HA + S <=> A- + SH+
In solution chemistry, it is common to use as an abbreviation for the solvated hydrogen ion, regardless of the solvent. In aqueous solution denotes a solvated hydronium ion rather than a proton.
The designation of an acid or base as "conjugate" depends on the context. The conjugate acid of a base B dissociates according to
:BH+ + OH- <=> B + H2O
which is the reverse of the equilibrium
:
The hydroxide ion , a well known base, is here acting as the conjugate base of the acid water. Acids and bases are thus regarded simply as donors and acceptors of protons respectively.
A broader definition of acid dissociation includes B(OH)3 + 2 H2O <=> B(OH)4- + H3O+
Similarly, metal ion hydrolysis causes ions such as to behave as weak acids:
Section 9.1 "Acidity of Solvated Cations" lists many p''K''a values.
: l(H2O)63+ + H2O <=>
According to
HA <=> A- + H+
The thermodynamic equilibrium constant can be defined by Chapter 2: Activity and Concentration Quotients
:
where represents the activity, at equilibrium, of the chemical species X. is  Since activity is the product of
Since activity is the product of
H2A <=> A^2- + 2H+
:
:
Note that in the context of metal-ligand complex formation, the equilibrium constants for the formation of metal complexes are usually defined as ''association'' constants. In that case, the equilibrium constants for ligand protonation are also defined as association constants. The numbering of association constants is the reverse of the numbering of dissociation constants; in this example
T equals the standard enthalpy change for the reaction divided by the product R times T squared. Here R represents the gas constant, which equals the thermal energy per mole per kelvin. The standard enthalpy is written as Delta H with a superscript plimsoll mark represented by the image strikeO. This equation follows from the definition of the Gibbs energy Delta G equals R times T times the natural logarithm of K.">
\frac = \frac
is the quantitative
Quantitative may refer to:
* Quantitative research, scientific investigation of quantitative properties
* Quantitative analysis (disambiguation)
* Quantitative verse, a metrical system in poetry
* Statistics, also known as quantitative analysis ...
measure of the strength
Strength may refer to:
Physical strength
*Physical strength, as in people or animals
* Hysterical strength, extreme strength occurring when people are in life-and-death situations
*Superhuman strength, great physical strength far above human c ...
of an acid in solution
Solution may refer to:
* Solution (chemistry), a mixture where one substance is dissolved in another
* Solution (equation), in mathematics
** Numerical solution, in numerical analysis, approximate solutions within specified error bounds
* Soluti ...
. It is the equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
for a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
:dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
in the context of acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica ...
s. The chemical species
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the wa ...
HA is an acid that dissociates into , the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of the acid and a hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle ...
, . The system is said to be in equilibrium when the concentrations of its components will not change over time, because both forward and backward reactions are occurring at the same rate.
The dissociation constant is defined by
: or
:
where quantities in square brackets represent the concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
s of the species at equilibrium.
Theoretical background
The acid dissociation constant for an acid is a direct consequence of the underlyingthermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
of the dissociation reaction; the p''K''a value is directly proportional to the standard Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and ...
change for the reaction. The value of the p''K''a changes with temperature and can be understood qualitatively based on Le Châtelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French ...
: when the reaction is endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
, ''K''a increases and p''K''a decreases with increasing temperature; the opposite is true for exothermic reactions.
The value of p''K''a also depends on molecular structure of the acid in many ways. For example, Pauling proposed two rules: one for successive p''K''a of polyprotic acids (see Polyprotic acids below), and one to estimate the p''K''a of oxyacids based on the number of =O and −OH groups (see Factors that affect p''K''a values below). Other structural factors that influence the magnitude of the acid dissociation constant include inductive effect
In chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond.
It is present in a σ (sigma ...
s, mesomeric effect
Mesomeric Effect in Organic Chemistry
The Mesomeric Effect
The mesomeric effect (or resonance effect) in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecu ...
s, and hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
. Hammett type equations have frequently been applied to the estimation of p''K''a.
The quantitative behaviour of acids and bases in solution can be understood only if their p''K''a values are known. In particular, the pH of a solution can be predicted when the analytical concentration and p''K''a values of all acids and bases are known; conversely, it is possible to calculate the equilibrium concentration of the acids and bases in solution when the pH is known. These calculations find application in many different areas of chemistry, biology, medicine, and geology. For example, many compounds used for medication are weak acids or bases, and a knowledge of the p''K''a values, together with the octanol-water partition coefficient
The ''n''-octanol-water partition coefficient, ''K''ow is a partition coefficient for the two-phase system consisting of ''n''-octanol and water. ''K''ow is also frequently referred to by the symbol P, especially in the English literature. It is a ...
, can be used for estimating the extent to which the compound enters the blood stream. Acid dissociation constants are also essential in aquatic chemistry
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be rep ...
and chemical oceanography
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The fiel ...
, where the acidity of water plays a fundamental role. In living organisms, acid–base homeostasis
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases (i.e. the pH) in the ECF is crucial for the normal physiology of the body—and for cellu ...
and enzyme kinetics
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in thi ...
are dependent on the p''K''a values of the many acids and bases present in the cell and in the body. In chemistry, a knowledge of p''K''a values is necessary for the preparation of buffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
s and is also a prerequisite for a quantitative understanding of the interaction between acids or bases and metal ions to form complexes. Experimentally, p''K''a values can be determined by potentiometric (pH) titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
, but for values of p''K''a less than about 2 or more than about 11, spectrophotometric or NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
measurements may be required due to practical difficulties with pH measurements.
Definitions
According to Arrhenius's original molecular definition, an acid is a substance that dissociates in aqueous solution, releasing the hydrogen ion (a proton): Chapter 6: Acid–Base and Donor–Acceptor Chemistry :acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica ...
:
: Brønsted and Lowry generalised this further to a proton exchange reaction: Includes discussion of many organic Brønsted acids.
Chapter 5: Acids and Bases
:
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide: the solvent S acts as a base, accepting a proton and forming the conjugate acid .
:
Brønsted and Lowry generalised this further to a proton exchange reaction: Includes discussion of many organic Brønsted acids.
Chapter 5: Acids and Bases
:
The acid loses a proton, leaving a conjugate base; the proton is transferred to the base, creating a conjugate acid. For aqueous solutions of an acid HA, the base is water; the conjugate base is and the conjugate acid is the hydronium ion. The Brønsted–Lowry definition applies to other solvents, such as dimethyl sulfoxide: the solvent S acts as a base, accepting a proton and forming the conjugate acid .
:hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
, in which protons are produced by the splitting of water molecules. For example, boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolve ...
() produces as if it were a proton donor, but it has been confirmed by Raman spectroscopy that this is due to the hydrolysis equilibrium:
:l(H2O)5(OH)
L, or l, is the twelfth letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''el'' (pronounced ), plural ''els''.
History
Lamedh ...
2+ + H3O+Lewis
Lewis may refer to:
Names
* Lewis (given name), including a list of people with the given name
* Lewis (surname), including a list of people with the surname
Music
* Lewis (musician), Canadian singer
* "Lewis (Mistreated)", a song by Radiohead ...
's original definition, an acid is a substance that accepts an electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he ...
to form a coordinate covalent bond.
p.698
Equilibrium constant
An acid dissociation constant is a particular example of anequilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
. The dissociation of a monoprotic acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
, HA, in dilute solution can be written as
: dimensionless
A dimensionless quantity (also known as a bare quantity, pure quantity, or scalar quantity as well as quantity of dimension one) is a quantity to which no physical dimension is assigned, with a corresponding SI unit of measurement of one (or 1) ...
since activity is dimensionless. Activities of the products of dissociation are placed in the numerator, activities of the reactants are placed in the denominator. See activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ( ...
for a derivation of this expression.
 Since activity is the product of
Since activity is the product of concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
and activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ( ...
(''γ'') the definition could also be written as
:
where represents the concentration of HA and is a quotient of activity coefficients.
To avoid the complications involved in using activities, dissociation constants are determined, where possible, in a medium of high ionic strength, that is, under conditions in which can be assumed to be always constant. For example, the medium might be a solution of 0.1 molar (M) sodium nitrate or 3 M potassium perchlorate. With this assumption,
:
:
is obtained. Note, however, that all published dissociation constant values refer to the specific ionic medium used in their determination and that different values are obtained with different conditions, as shown for acetic acid in the illustration above. When published constants refer to an ionic strength other than the one required for a particular application, they may be adjusted by means of specific ion theory (SIT) and other theories.
Cumulative and stepwise constants
A cumulative equilibrium constant, denoted by is related to the product of stepwise constants, denoted by For a dibasic acid the relationship between stepwise and overall constants is as follows :Association and dissociation constants
When discussing the properties of acids it is usual to specify equilibrium constants as acid dissociation constants, denoted by ''K''a, with numerical values given the symbol p''K''a. : On the other hand, association constants are used for bases. : However, general purpose computer programs that are used to derive equilibrium constant values from experimental data use association constants for both acids and bases. Because stability constants for a metal-ligand complex are always specified as association constants, ligand protonation must also be specified as an association reaction. The definitions show that the value of an acid dissociation constant is the reciprocal of the value of the corresponding association constant. : : : Notes # For a given acid or base , the self-ionization constant of water. # The association constant for the formation of asupramolecular
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces ...
complex may be denoted as Ka; in such cases "a" stands for "association", not "acid".
# For polyprotic acids, the numbering of stepwise association constants is the reverse of the numbering of the dissociation constants. For example, for phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
(details in #polyprotic acids, below)
::
Temperature dependence
All equilibrium constants vary withtemperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
according to the van 't Hoff equation
The Van 't Hoff equation relates the change in the equilibrium constant, , of a chemical reaction to the change in temperature, ''T'', given the standard enthalpy change, , for the process. It was proposed by Dutch chemist Jacobus Henricus van ' ...
:gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and is the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic w ...
. Thus, for exothermic reactions, the standard enthalpy change
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
, , is negative and ''K'' decreases with temperature. For endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
reactions, is positive and ''K'' increases with temperature.
The standard enthalpy change for a reaction is itself a function of temperature, according to Kirchhoff's law of thermochemistry:
:
where is the heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
change at constant pressure. In practice may be taken to be constant over a small temperature range.
Dimensionality
In the equation : ''K''a appears to havedimensions
In physics and mathematics, the dimension of a mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any point within it. Thus, a line has a dimension of one (1D) because only one coordin ...
of concentration. However, since , the equilibrium constant, , ''cannot'' have a physical dimension. This apparent paradox can be resolved in various ways.
# Assume that the quotient of activity coefficients has a numerical value of 1, so that has the same numerical value as the thermodynamic equilibrium constant .
# Express each concentration value as the ratio c/c0, where c0 is the concentration in a ypotheticalstandard state, with a numerical value of 1, by definition.
# Express the concentrations on the mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ex ...
scale. Since mole fraction has no dimension, the quotient of concentrations will, by definition, be a pure number.
The procedures, (1) and (2), give identical numerical values for an equilibrium constant. Furthermore, since a concentration is simply proportional to mole fraction and density :
:
and since the molar mass is a constant in dilute solutions, an equilibrium constant value determined using (3) will be simply proportional to the values obtained with (1) and (2).
It is common practice in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
to quote a value with a dimension as, for example, "''K''a = 30 mM" in order to indicate the scale, millimolar (mM) or micromolar (μM) of the concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
values used for its calculation.
Strong acids and bases
An acid is classified as "strong" when the concentration of its undissociated species is too low to be measured. Any aqueous acid with a p''K''a value of less than 0 is almost completely deprotonated and is considered a ''strong acid''. Sec. 5.1c Strong and weak acids and bases All such acids transfer their protons to water and form the solvent cation species (H3O+ in aqueous solution) so that they all have essentially the same acidity, a phenomenon known as solvent leveling. Sec. 5.2 Solvent leveling They are said to be ''fully dissociated'' in aqueous solution because the amount of undissociated acid, in equilibrium with the dissociation products, is below thedetection limit
The limit of detection (LOD or LoD) is the lowest signal, or the lowest corresponding quantity to be determined (or extracted) from the signal, that can be observed with a sufficient degree of confidence or statistical significance. However, the ...
.
Likewise, any aqueous base with an association constant
The binding constant, or affinity constant/association constant, is a special case of the equilibrium constant ''K'', and is the inverse of the dissociation constant. It is associated with the binding and unbinding reaction of receptor (R) and li ...
p''K''b less than about 0, corresponding to p''K''a greater than about 14, is leveled to OH− and is considered a ''strong base''.
Nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, with a p''K'' value of ca. -1.7, behaves as a strong acid in aqueous solutions with a pH greater than 1. At lower pH values it behaves as a weak acid.
p''K''a values for strong acids have been estimated by theoretical means. For example, the p''K''a value of aqueous HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a spe ...
has been estimated as −9.3.
Monoprotic acids
Henderson–Hasselbalch equation
In chemistry and biochemistry, the Henderson–Hasselbalch equation
:\ce = \ceK_\ce + \log_ \left( \frac \right)
relates the pH of a chemical solution of a weak acid to the numerical value of the acid dissociation constant, ''K''a, of acid and th ...
, from which the following conclusions can be drawn.
* At half-neutralization the ratio ; since , the pH at half-neutralization is numerically equal to p''K''a. Conversely, when , the concentration of HA is equal to the concentration of A−.
* The buffer region extends over the approximate range p''K''a ± 2. Buffering is weak outside the range p''K''a ± 1. At pH ≤ p''K''a − 2 the substance is said to be fully protonated and at pH ≥ p''K''a + 2 it is fully dissociated (deprotonated).
* If the pH is known, the ratio may be calculated. This ratio is independent of the analytical concentration of the acid.
In water, measurable p''K''a values range from about −2 for a strong acid to about 12 for a very weak acid (or strong base).
A buffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
of a desired pH can be prepared as a mixture of a weak acid and its conjugate base. In practice, the mixture can be created by dissolving the acid in water, and adding the requisite amount of strong acid or base. When the p''K''a and analytical concentration of the acid are known, the extent of dissociation and pH of a solution of a monoprotic acid can be easily calculated using an ICE table.
Polyprotic acids
 A polyprotic acid is a compound which may lose more than 1 proton. Stepwise dissociation constants are each defined for the loss of a single proton. The constant for dissociation of the first proton may be denoted as ''K''a1 and the constants for dissociation of successive protons as ''K''a2, etc.
A polyprotic acid is a compound which may lose more than 1 proton. Stepwise dissociation constants are each defined for the loss of a single proton. The constant for dissociation of the first proton may be denoted as ''K''a1 and the constants for dissociation of successive protons as ''K''a2, etc. Phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, w ...
, , is an example of a polyprotic acid as it can lose three protons.
:
When the difference between successive p''K'' values is about four or more, as in this example, each species may be considered as an acid in its own right; In fact salts of may be crystallised from solution by adjustment of pH to about 5.5 and salts of may be crystallised from solution by adjustment of pH to about 10. The species distribution diagram shows that the concentrations of the two ions are maximum at pH 5.5 and 10.
 When the difference between successive p''K'' values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
According to Pauling's first rule, successive p''K'' values of a given acid increase . For oxyacids with more than one ionizable hydrogen on the same atom, the p''K''a values often increase by about 5 units for each proton removed, as in the example of phosphoric acid above.
It can be seen in the table above that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it. That why p''K''a2 is greater than p''K''a1. p''K''a3 is greater than p''K''a2 because there is further charge separation. When an exception to Pauling's rule is found, it indicates that a major change in structure is also occurring. In the case of (aq), the vanadium is
When the difference between successive p''K'' values is less than about four there is overlap between the pH range of existence of the species in equilibrium. The smaller the difference, the more the overlap. The case of citric acid is shown at the right; solutions of citric acid are buffered over the whole range of pH 2.5 to 7.5.
According to Pauling's first rule, successive p''K'' values of a given acid increase . For oxyacids with more than one ionizable hydrogen on the same atom, the p''K''a values often increase by about 5 units for each proton removed, as in the example of phosphoric acid above.
It can be seen in the table above that the second proton is removed from a negatively charged species. Since the proton carries a positive charge extra work is needed to remove it. That why p''K''a2 is greater than p''K''a1. p''K''a3 is greater than p''K''a2 because there is further charge separation. When an exception to Pauling's rule is found, it indicates that a major change in structure is also occurring. In the case of (aq), the vanadium is octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet a ...
, 6-coordinate, whereas vanadic acid is tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
, 4-coordinate. This means that four "particles" are released with the first dissociation, but only two "particles" are released with the other dissociations, resulting in a much greater entropy contribution to the standard Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and ...
change for the first reaction than for the others.
:
Isoelectric point
For substances in solution, the isoelectric point (p''I'') is defined as the pH at which the sum, weighted by charge value, of concentrations of positively charged species is equal to the weighted sum of concentrations of negatively charged species. In the case that there is one species of each type, the isoelectric point can be obtained directly from the p''K'' values. Take the example ofglycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
, defined as AH. There are two dissociation equilibria to consider.
: cologarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 o ...
s, the pH is given by
:
p''I'' values for amino acids are listed at proteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino aci ...
. When more than two charged species are in equilibrium with each other a full speciation calculation may be needed.
Bases and basicity
The equilibrium constant ''K''b for a base is usually defined as the ''association'' constant for protonation of the base, B, to form the conjugate acid, . :hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ion, , is related to the concentration of the hydrogen ion by , therefore
:
Substitution of the expression for into the expression for ''K''b gives
:
When ''K''a, ''K''b and ''K''w are determined under the same conditions of temperature and ionic strength, it follows, taking cologarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 o ...
s, that p''K''b = p''K''w − p''K''a. In aqueous solutions at 25 °C, p''K''w is 13.9965, Section D–152 so
:
with sufficient accuracy
Accuracy and precision are two measures of ''observational error''.
''Accuracy'' is how close a given set of measurements ( observations or readings) are to their ''true value'', while ''precision'' is how close the measurements are to each oth ...
for most practical purposes. In effect there is no need to define p''K''b separately from p''K''a, but it is done here as often only p''K''b values can be found in the older literature.
For an hydrolyzed metal ion, ''K''b can also be defined as a stepwise ''dissociation'' constant
:
:
This is the reciprocal of an association constant
The binding constant, or affinity constant/association constant, is a special case of the equilibrium constant ''K'', and is the inverse of the dissociation constant. It is associated with the binding and unbinding reaction of receptor (R) and li ...
for formation of the complex.
Basicity expressed as dissociation constant of conjugate acid
Because the relationship p''K''b = p''K''w − p''K''a holds only in aqueous solutions (though analogous relationships apply for other amphoteric solvents), subdisciplines of chemistry likeorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
that usually deal with nonaqueous solutions generally do not use p''K''b as a measure of basicity. Instead, the p''K''a of the conjugate acid, denoted by p''K''aH, is quoted when basicity needs to be quantified. For base B and its conjugate acid BH+ in equilibrium, this is defined as
:
A higher value for p''K''aH corresponds to a stronger base. For example, the values and indicate that (triethylamine) is a stronger base than (pyridine).
Amphoteric substances
Anamphoteric
In chemistry, an amphoteric compound () is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.
One type of amphoteric species are amphipro ...
substance is one that can act as an acid or as a base, depending on pH. Water (below) is amphoteric. Another example of an amphoteric molecule is the bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochem ...
ion that is the conjugate base of the carbonic acid molecule H2CO3 in the equilibrium
:carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
ion in (the reverse of) the equilibrium
:acid–base homeostasis
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases (i.e. the pH) in the ECF is crucial for the normal physiology of the body—and for cellu ...
in the human body.
An amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
is also amphoteric with the added complication that the neutral molecule is subject to an internal acid–base equilibrium in which the basic amino group attracts and binds the proton from the acidic carboxyl group, forming a zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
.
: Water self-ionization
The water molecule may either gain or lose a proton. It is said to be amphiprotic. The ionization equilibrium can be written :hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
ion , but this formula is not exact because in fact there is solvation by more than one water molecule and species such as , , and are also present.
The equilibrium constant is given by
:
With solutions in which the solute concentrations are not very high, the concentration can be assumed to be constant, regardless of solute(s); this expression may then be replaced by
:
The self-ionization constant of water, ''K''w, is thus just a special case of an acid dissociation constant. A logarithmic form analogous to p''K''a may also be defined
:
These data can be modelled by a parabola
In mathematics, a parabola is a plane curve which is Reflection symmetry, mirror-symmetrical and is approximately U-shaped. It fits several superficially different Mathematics, mathematical descriptions, which can all be proved to define exact ...
with
:
From this equation, p''K''w = 14 at 24.87 °C. At that temperature both hydrogen and hydroxide ions have a concentration of 10−7 M.
Acidity in nonaqueous solutions
A solvent will be more likely to promote ionization of a dissolved acidic molecule in the following circumstances: # It is aprotic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
, capable of forming hydrogen bonds.
# It has a high donor number In chemistry a donor number (DN) is a quantitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 (antimony pentachloride), in ...
, making it a strong Lewis base.
# It has a high dielectric constant (relative permittivity), making it a good solvent for ionic species.
p''K''a values of organic compounds are often obtained using the aprotic solvents dimethyl sulfoxide (DMSO) and acetonitrile (ACN).
DMSO is widely used as an alternative to water because it has a lower dielectric constant than water, and is less polar and so dissolves non-polar, hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
substances more easily. It has a measurable p''K''a range of about 1 to 30. Acetonitrile is less basic than DMSO, and, so, in general, acids are weaker and bases are stronger in this solvent. Some p''K''a values at 25 °C for acetonitrile (ACN) and dimethyl sulfoxide (DMSO). are shown in the following tables. Values for water are included for comparison.
Ionization of acids is less in an acidic solvent than in water. For example, hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
is a weak acid when dissolved in acetic acid. This is because acetic acid is a much weaker base than water.
:  The unlikely
The unlikely geminal diol
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached t ...
species is stable in these environments. For aqueous solutions the pH scale is the most convenient acidity function. Other acidity functions have been proposed for non-aqueous media, the most notable being the Hammett acidity function, ''H''0, for superacid media and its modified version ''H''− for superbasic
SuperBASIC is an advanced variant of the BASIC programming language with many structured programming additions. It was developed at Sinclair Research by Jan Jones during the early 1980s. Originally SuperBASIC was intended as the BASIC interprete ...
media.
In aprotic solvents, oligomers, such as the well-known acetic acid dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
, may be formed by hydrogen bonding. An acid may also form hydrogen bonds to its conjugate base. This process, known as homoconjugation, has the effect of enhancing the acidity of acids, lowering their effective p''K''a values, by stabilizing the conjugate base. Homoconjugation enhances the proton-donating power of toluenesulfonic acid in acetonitrile solution by a factor of nearly 800.
In aqueous solutions, homoconjugation does not occur, because water forms stronger hydrogen bonds to the conjugate base than does the acid.
Mixed solvents
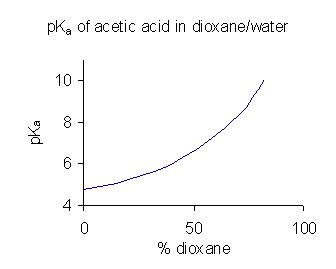
 When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine p''K''a values in a solvent mixture such as water/
When a compound has limited solubility in water it is common practice (in the pharmaceutical industry, for example) to determine p''K''a values in a solvent mixture such as water/dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
or water/ methanol, in which the compound is more soluble. In the example shown at the right, the p''K''a value rises steeply with increasing percentage of dioxane as the dielectric constant of the mixture is decreasing.
A p''K''a value obtained in a mixed solvent cannot be used directly for aqueous solutions. The reason for this is that when the solvent is in its standard state its activity is ''defined'' as one. For example, the standard state of water:dioxane mixture with 9:1 mixing ratio is precisely that solvent mixture, with no added solutes. To obtain the p''K''a value for use with aqueous solutions it has to be extrapolated to zero co-solvent concentration from values obtained from various co-solvent mixtures.
These facts are obscured by the omission of the solvent from the expression that is normally used to define p''K''a, but p''K''a values obtained in a ''given'' mixed solvent can be compared to each other, giving relative acid strengths. The same is true of p''K''a values obtained in a particular non-aqueous solvent such a DMSO.
A universal, solvent-independent, scale for acid dissociation constants has not been developed, since there is no known way to compare the standard states of two different solvents.
Factors that affect p''K''a values
Pauling's second rule is that the value of the first p''K''a for acids of the formula XO''m''(OH)''n'' depends primarily on the number of oxo groups ''m'', and is approximately independent of the number of hydroxy groups ''n'', and also of the central atom X. Approximate values of p''K''a are 8 for ''m'' = 0, 2 for ''m'' = 1, −3 for ''m'' = 2 and < −10 for ''m'' = 3. Alternatively, various numerical formulas have been proposed including p''K''a = 8 − 5''m'' (known as Bell's rule), p''K''a = 7 − 5''m'',Douglas B., McDaniel D.H. and Alexander J.J. ''Concepts and Models of Inorganic Chemistry'' (2nd ed. Wiley 1983) p.526 or p''K''a = 9 − 7''m''. The dependence on ''m'' correlates with the oxidation state of the central atom, X: the higher the oxidation state the stronger the oxyacid. For example, p''K''a for HClO is 7.2, for HClO2 is 2.0, for HClO3 is −1 and HClO4 is a strong acid (). The increased acidity on adding an oxo group is due to stabilization of the conjugate base by delocalization of its negative charge over an additional oxygen atom. This rule can help assign molecular structure: for example,phosphorous acid
Phosphorous acid (or phosphonic acid (singular)) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in th ...
(H3PO3) has a p''K''a near 2, which suggested that the structure is HPO(OH)2, as later confirmed by NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
, and not P(OH)3, which would be expected to have a p''K''a near 8.

mesomeric effect
Mesomeric Effect in Organic Chemistry
The Mesomeric Effect
The mesomeric effect (or resonance effect) in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecu ...
s affect the p''K''a values. A simple example is provided by the effect of replacing the hydrogen atoms in acetic acid by the more electronegative chlorine atom. The electron-withdrawing effect of the substituent makes ionisation easier, so successive p''K''a values decrease in the series 4.7, 2.8, 1.4, and 0.7 when 0, 1, 2, or 3 chlorine atoms are present. The Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with ju ...
, provides a general expression for the effect of substituents.
: log(''K''a) = log(''K'') + ρσ.
''K''a is the dissociation constant of a substituted compound, ''K'' is the dissociation constant when the substituent is hydrogen, ρ is a property of the unsubstituted compound and σ has a particular value for each substituent. A plot of log(''K''a) against σ is a straight line with intercept log(''K'') and slope
In mathematics, the slope or gradient of a line is a number that describes both the ''direction'' and the ''steepness'' of the line. Slope is often denoted by the letter ''m''; there is no clear answer to the question why the letter ''m'' is use ...
ρ. This is an example of a linear free energy relationship as log(''K''a) is proportional to the standard free energy change. Hammett originally formulated the relationship with data from benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, ...
with different substituents in the '' ortho-'' and ''para
Para, or PARA, may refer to:
Businesses and organizations
* Paramount Global, traded as PARA on the Nasdaq stock exchange
* Para Group, the former name of CT Corp
* Para Rubber, now Skellerup, a New Zealand manufacturer
* Para USA, formerly ...
-'' positions: some numerical values are in Hammett equation
The Hammett equation in organic chemistry describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with ju ...
. This and other studies allowed substituents to be ordered according to their electron-withdrawing
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of th ...
or electron-releasing power, and to distinguish between inductive and mesomeric effects.
Alcohols do not normally behave as acids in water, but the presence of a double bond adjacent to the OH group can substantially decrease the p''K''a by the mechanism of keto–enol tautomerism
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The t ...
. Ascorbic acid is an example of this effect. The diketone 2,4-pentanedione ( acetylacetone) is also a weak acid because of the keto–enol equilibrium. In aromatic compounds, such as phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
, which have an OH substituent, conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
with the aromatic ring as a whole greatly increases the stability of the deprotonated form.
Structural effects can also be important. The difference between fumaric acid
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as fu ...
and maleic acid
Maleic acid or ''cis''-butenedioic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Its chemical formula is HO2CCH=CHCO2H. Maleic acid is the ''cis''-isomer of butenedioic acid, whereas fumaric ac ...
is a classic example. Fumaric acid is (E)-1,4-but-2-enedioic acid, a ''trans'' isomer, whereas maleic acid is the corresponding ''cis'' isomer, i.e. (Z)-1,4-but-2-enedioic acid (see cis-trans isomerism). Fumaric acid has p''K''a values of approximately 3.0 and 4.5. By contrast, maleic acid has p''K''a values of approximately 1.5 and 6.5. The reason for this large difference is that when one proton is removed from the ''cis'' isomer (maleic acid) a strong intramolecular hydrogen bond is formed with the nearby remaining carboxyl group. This favors the formation of the maleate H+, and it opposes the removal of the second proton from that species. In the ''trans'' isomer, the two carboxyl groups are always far apart, so hydrogen bonding is not observed.
Proton sponge
1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula CH(NMe) (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional ...
, 1,8-bis(dimethylamino)naphthalene, has a p''K''a value of 12.1. It is one of the strongest amine bases known. The high basicity is attributed to the relief of strain upon protonation and strong internal hydrogen bonding.
Effects of the solvent and solvation should be mentioned also in this section. It turns out, these influences are more subtle than that of a dielectric medium mentioned above. For example, the expected (by electronic effects of methyl substituents) and observed in gas phase order of basicity of methylamines, Me3N > Me2NH > MeNH2 > NH3, is changed by water to Me2NH > MeNH2 > Me3N > NH3. Neutral methylamine molecules are hydrogen-bonded to water molecules mainly through one acceptor, N–HOH, interaction and only occasionally just one more donor bond, NH–OH2. Hence, methylamines are stabilized to about the same extent by hydration, regardless of the number of methyl groups. In stark contrast, corresponding methylammonium cations always utilize all the available protons for donor NH–OH2 bonding. Relative stabilization of methylammonium ions thus decreases with the number of methyl groups explaining the order of water basicity of methylamines.
Thermodynamics
An equilibrium constant is related to the standardGibbs energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pre ...
change for the reaction, so for an acid dissociation constant
: .
''R'' is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and ''T'' is the absolute temperature
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Historically, thermodynamic temperature was defined by Kelvin in terms of a macroscopic relation between thermodynamic w ...
. Note that and . At 25 °C, Δ''G'' in kJ·mol−1 ≈ 5.708 p''K''a (1 kJ·mol−1 = 1000 joule
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applie ...
s per mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
). Free energy is made up of an enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
term and an entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
term.
:
The standard enthalpy change can be determined by calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in ''state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reac ...
or by using the van 't Hoff equation
The Van 't Hoff equation relates the change in the equilibrium constant, , of a chemical reaction to the change in temperature, ''T'', given the standard enthalpy change, , for the process. It was proposed by Dutch chemist Jacobus Henricus van ' ...
, though the calorimetric method is preferable. When both the standard enthalpy change and acid dissociation constant have been determined, the standard entropy change is easily calculated from the equation above. In the following table, the entropy terms are calculated from the experimental values of p''K''a and Δ''H''. The data were critically selected and refer to 25 °C and zero ionic strength, in water.
The first point to note is that, when p''K''a is positive, the standard free energy change for the dissociation reaction is also positive. Second, some reactions are exothermic and some are endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
, but, when Δ''H'' is negative ''T''ΔS is the dominant factor, which determines that Δ''G'' is positive. Last, the entropy contribution is always unfavourable () in these reactions. Ions in aqueous solution tend to orient the surrounding water molecules, which orders the solution and decreases the entropy. The contribution of an ion to the entropy is the partial molar entropy which is often negative, especially for small or highly charged ions. The ionization of a neutral acid involves formation of two ions so that the entropy decreases (). On the second ionization of the same acid, there are now three ions and the anion has a charge, so the entropy again decreases.
Note that the ''standard'' free energy change for the reaction is for the changes ''from'' the reactants in their standard states ''to'' the products in their standard states. The free energy change ''at'' equilibrium is zero since the chemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species ...
s of reactants and products are equal at equilibrium.
Experimental determination
 The experimental determination of p''K''a values is commonly performed by means of
The experimental determination of p''K''a values is commonly performed by means of titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
s, in a medium of high ionic strength and at constant temperature. A typical procedure would be as follows. A solution of the compound in the medium is acidified with a strong acid to the point where the compound is fully protonated. The solution is then titrated with a strong base until all the protons have been removed. At each point in the titration pH is measured using a glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an exampl ...
and a pH meter
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH elect ...
. The equilibrium constants are found by fitting calculated pH values to the observed values, using the method of least squares.
The total volume of added strong base should be small compared to the initial volume of titrand solution in order to keep the ionic strength nearly constant. This will ensure that p''K''a remains invariant during the titration.
A calculated titration curve
Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the pH of the solution as the dependent variable (because it changes depending on the composition ...
for oxalic acid is shown at the right. Oxalic acid has p''K''a values of 1.27 and 4.27. Therefore, the buffer regions will be centered at about pH 1.3 and pH 4.3. The buffer regions carry the information necessary to get the p''K''a values as the concentrations of acid and conjugate base change along a buffer region.
Between the two buffer regions there is an end-point, or equivalence point
The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been mixed. For an acid-base reaction the equivalence point is where the moles of acid and the moles of ...
, at about pH 3. This end-point is not sharp and is typical of a diprotic acid whose buffer regions overlap by a small amount: p''K''a2 − p''K''a1 is about three in this example. (If the difference in p''K'' values were about two or less, the end-point would not be noticeable.) The second end-point begins at about pH 6.3 and is sharp. This indicates that all the protons have been removed. When this is so, the solution is not buffered and the pH rises steeply on addition of a small amount of strong base. However, the pH does not continue to rise indefinitely. A new buffer region begins at about pH 11 (p''K''w − 3), which is where self-ionization of water
The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen ...
becomes important.
It is very difficult to measure pH values of less than two in aqueous solution with a glass electrode
A glass electrode is a type of ion-selective electrode made of a doped glass membrane that is sensitive to a specific ion. The most common application of ion-selective glass electrodes is for the measurement of pH. The pH electrode is an exampl ...
, because the Nernst equation
In electrochemistry, the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction ( half-cell or full cell reaction) from the standard electrode potential, absolute tempe ...
breaks down at such low pH values. To determine p''K'' values of less than about 2 or more than about 11 spectrophotometric or NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
measurements may be used instead of, or combined with, pH measurements.
When the glass electrode cannot be employed, as with non-aqueous solutions, spectrophotometric methods are frequently used. These may involve absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative lo ...
or fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
measurements. In both cases the measured quantity is assumed to be proportional to the sum of contributions from each photo-active species; with absorbance measurements the Beer–Lambert law
The Beer–Lambert law, also known as Beer's law, the Lambert–Beer law, or the Beer–Lambert–Bouguer law relates the attenuation of light to the properties of the material through which the light is travelling. The law is commonly applied t ...
is assumed to apply.
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules (such as medicinal compounds) to larger macro ...
(ITC) may be used to determine both a p''K'' value and the corresponding standard enthalpy for acid dissociation. Software to perform the calculations is supplied by the instrument manufacturers for simple systems.
Aqueous solutions with normal water cannot be used for 1H NMR measurements but heavy water, , must be used instead. 13C NMR data, however, can be used with normal water and 1H NMR spectra can be used with non-aqueous media. The quantities measured with NMR are time-averaged chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure o ...
s, as proton exchange is fast on the NMR time-scale. Other chemical shifts, such as those of 31P can be measured.
Micro-constants
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
cysteine, which exists in solution as a neutral zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
. The two microconstants represent deprotonation either at sulphur or at nitrogen, and the macroconstant sum here is the acid dissociation constant
spermine
Spermine is a polyamine involved in cellular metabolism that is found in all eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some bacteria as well. It is found as a p ...
has more than one site where protonation can occur. For example, monoprotonation can occur at a terminal group or at internal groups. The ''K''b values for dissociation of spermine protonated at one or other of the sites are examples of micro-constants. They cannot be determined directly by means of pH, absorbance, fluorescence or NMR measurements; a measured ''K''b value is the sum of the K values for the micro-reactions.
:
Nevertheless, the site of protonation is very important for biological function, so mathematical methods have been developed for the determination of micro-constants.
When two reactants form a single product in parallel, the macroconstant For example, the abovementioned equilibrium for spermine may be considered in terms of ''K''a values of two tautomeric
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
conjugate acids, with macroconstant In this case This is equivalent to the preceding expression since is proportional to
When a reactant undergoes two reactions in series, the macroconstant for the combined reaction is the product of the microconstant for the two steps. For example, the abovementioned cysteine zwitterion can lose two protons, one from sulphur and one from nitrogen, and the overall macroconstant for losing two protons is the product of two dissociation constants This can also be written in terms of logarithmic constants as
Applications and significance
A knowledge of p''K''a values is important for the quantitative treatment of systems involving acid–base equilibria in solution. Many applications exist inbiochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
; for example, the p''K''a values of proteins and amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
side chains are of major importance for the activity of enzymes and the stability of proteins. Protein p''K''a values cannot always be measured directly, but may be calculated using theoretical methods. Buffer solutions
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
are used extensively to provide solutions at or near the physiological pH for the study of biochemical reactions; the design of these solutions depends on a knowledge of the p''K''a values of their components. Important buffer solutions include MOPS
MOPS (3-(''N''-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. It is a structural analog to MES, and like MES, its structure contains a morpholine ring. HEPES is a similar pH buffering ...
, which provides a solution with pH 7.2, and tricine
Tricine is an organic compound that is used in buffer solutions. The name tricine comes from tris and glycine, from which it was derived.Good, N.E., et al., Biochemistry, v. 5, 467 (1966). It is a white crystalline powder that is moderately solub ...
, which is used in gel electrophoresis. Buffering is an essential part of acid base physiology including acid–base homeostasis
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases (i.e. the pH) in the ECF is crucial for the normal physiology of the body—and for cellu ...
, and is key to understanding disorders such as acid–base disorder
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range (7.35 to 7.45). In the fetus, the normal range differs based on which umbilical vessel i ...
. The isoelectric point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also u ...
of a given molecule is a function of its p''K'' values, so different molecules have different isoelectric points. This permits a technique called isoelectric focusing
Isoelectric focusing (IEF), also known as electrofocusing, is a technique for separating different molecules by differences in their isoelectric point (pI). It is a type of zone electrophoresis usually performed on proteins in a gel that takes ad ...
, which is used for separation of proteins by 2-D gel polyacrylamide gel electrophoresis.
Buffer solutions also play a key role in analytical chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separati ...
. They are used whenever there is a need to fix the pH of a solution at a particular value. Compared with an aqueous solution, the pH of a buffer solution is relatively insensitive to the addition of a small amount of strong acid or strong base. The buffer capacity of a simple buffer solution is largest when pH = p''K''a. In acid–base extraction
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds. It is typically performed during the work-up step following a chemical synthesis to puri ...
, the efficiency of extraction of a compound into an organic phase, such as an ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
, can be optimised by adjusting the pH of the aqueous phase using an appropriate buffer. At the optimum pH, the concentration of the electrically neutral species is maximised; such a species is more soluble in organic solvents having a low dielectric constant than it is in water. This technique is used for the purification of weak acids and bases.
A pH indicator
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, ...
is a weak acid or weak base that changes colour in the transition pH range, which is approximately p''K''a ± 1. The design of a universal indicator
__FORCETOC__
A universal indicator is a pH indicator made of a solution of several compounds that exhibits several smooth colour changes over a wide range pH values to indicate the acidity or alkalinity of solutions. Although there are several ...
requires a mixture of indicators whose adjacent p''K''a values differ by about two, so that their transition pH ranges just overlap.
In pharmacology, ionization of a compound alters its physical behaviour and macro properties such as solubility and lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lip ...
, log ''p''). For example, ionization of any compound will increase the solubility in water, but decrease the lipophilicity. This is exploited in drug development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for re ...
to increase the concentration of a compound in the blood by adjusting the p''K''a of an ionizable group.
Knowledge of p''K''a values is important for the understanding of coordination complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
, which are formed by the interaction of a metal ion, Mm+, acting as a Lewis acid, with a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
, L, acting as a Lewis base. However, the ligand may also undergo protonation reactions, so the formation of a complex in aqueous solution could be represented symbolically by the reaction
:
To determine the equilibrium constant for this reaction, in which the ligand loses a proton, the p''K''a of the protonated ligand must be known. In practice, the ligand may be polyprotic; for example EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
4− can accept four protons; in that case, all p''K''a values must be known. In addition, the metal ion is subject to hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
, that is, it behaves as a weak acid, so the p''K'' values for the hydrolysis reactions must also be known.
Assessing the hazard associated with an acid or base may require a knowledge of p''K''a values. For example, hydrogen cyanide is a very toxic gas, because the cyanide ion inhibits the iron-containing enzyme cytochrome c oxidase
The enzyme cytochrome c oxidase or Complex IV, (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and mitochondria of eukaryotes.
It is the last enzyme in the respiratory elect ...
. Hydrogen cyanide is a weak acid in aqueous solution with a p''K''a of about 9. In strongly alkaline solutions, above pH 11, say, it follows that sodium cyanide is "fully dissociated" so the hazard due to the hydrogen cyanide gas is much reduced. An acidic solution, on the other hand, is very hazardous because all the cyanide is in its acid form. Ingestion of cyanide by mouth is potentially fatal, independently of pH, because of the reaction with cytochrome c oxidase.
In environmental science acid–base equilibria are important for lakes and rivers; for example, humic acids are important components of natural waters. Another example occurs in chemical oceanography
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The fiel ...
: in order to quantify the solubility of iron(III) in seawater at various salinities
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
, the p''K''a values for the formation of the iron(III) hydrolysis products , and were determined, along with the solubility product Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reacti ...
of iron hydroxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of whi ...
.
Values for common substances
There are multiple techniques to determine the p''K''a of a chemical, leading to some discrepancies between different sources. Well measured values are typically within 0.1 units of each other. Data presented here were taken at 25 °C in water. Chapter 8 More values can be found in theThermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
section, above. A table of p''K''a of carbon acids, measured in DMSO, can be found on the page on carbanions.
See also
*Acidosis
Acidosis is a process causing increased acidity in the blood and other body tissues (i.e., an increase in hydrogen ion concentration). If not further qualified, it usually refers to acidity of the blood plasma.
The term ''acidemia'' describes ...
* Acids in wine: tartaric, malic and citric are the principal acids in wine.
* Alkalosis
Alkalosis is the result of a process reducing hydrogen ion concentration of arterial blood plasma (alkalemia). In contrast to acidemia (serum pH 7.35 or lower), alkalemia occurs when the serum pH is higher than normal (7.45 or higher). Alkalosis ...
* Arterial blood gas
An arterial blood gas (ABG) test, or arterial blood gas analysis (ABGA) measures the amounts of arterial gases, such as oxygen and carbon dioxide. An ABG test requires that a small volume of blood be drawn from the radial artery with a syringe an ...
* Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the Reagent, reactants and Product (chemistry), products are present in concentrations which have no further tendency to change with time, so that there is no observable chan ...
* Conductivity (electrolytic)
* Grotthuss mechanism: how protons are transferred between hydronium ions and water molecules, accounting for the exceptionally high ionic mobility of the proton (animation).
* Hammett acidity function: a measure of acidity that is used for very concentrated solutions of strong acids, including superacids.
* Ion transport number
* Ocean acidification: dissolution of atmospheric carbon dioxide affects pH#Seawater, seawater pH. The reaction depends on total inorganic carbon and on solubility equilibria with solid carbonates such as limestone and dolomite (mineral), dolomite.
* Law of dilution
* pCO2
* pH
* Predominance diagram: relates to equilibria involving oxyanion, polyoxyanions. p''K''a values are needed to construct these diagrams.
* Proton affinity: a measure of basicity in the gas phase.
* Stability constants of complexes: formation of a complex can often be seen as a competition between proton and metal ion for a ligand, which is the product of dissociation of an acid.
Notes
References
Further reading
* (Previous edition published as ) * * (Non-aqueous solvents) * (translation editor: Mary R. Masson) * * Chapter 4: Solvent Effects on the Position of Homogeneous Chemical Equilibria. *External links
Acidity–Basicity Data in Nonaqueous Solvents
Extensive bibliography of p''K''a values in dimethyl sulfoxide, DMSO, acetonitrile, THF, heptane, 1,2-dichloroethane, and in the gas phase
Curtipot
All-in-one freeware for pH and acid–base equilibrium calculations and for simulation and analysis of potentiometric titration curves with spreadsheets
SPARC Physical/Chemical property calculator
Includes a database with aqueous, non-aqueous, and gaseous phase p''K''a values than can be searched using Simplified molecular input line entry specification, SMILES or CAS registry numbers
Aqueous-Equilibrium Constants
p''K''a values for various acid and bases. Includes a table of some solubility products
Explanations of the relevance of these properties to pharmacology
Free online prediction tool (Marvin)
p''K''a, log ''p'', log ''d'' etc. From Chemaxon, ChemAxon * Chemicalize.org:Chemicalize.org#List of the predicted structure based properties, List of predicted structure based properties * p''K''a Char
by David A. Evans {{Chemical solutions Equilibrium chemistry Acids Bases (chemistry) Analytical chemistry Physical chemistry